Eliminating risk of toxic drug exposure
Even for well-established anti-cancer drugs, it is crucial that their manufacturers understand the current and evolving handling practices at hospital pharmacies since these compounds can be highly toxic and volatile.
Bristol Myers Squibb sought to gain such understanding with their popular Taxol® cytotoxic drug – with the goal of adding value by addressing challenges that hospitals may encounter with this product. PDD’s research across several European markets revealed several issues during preparation for administration, despite the procedure being performed in a safety chamber.
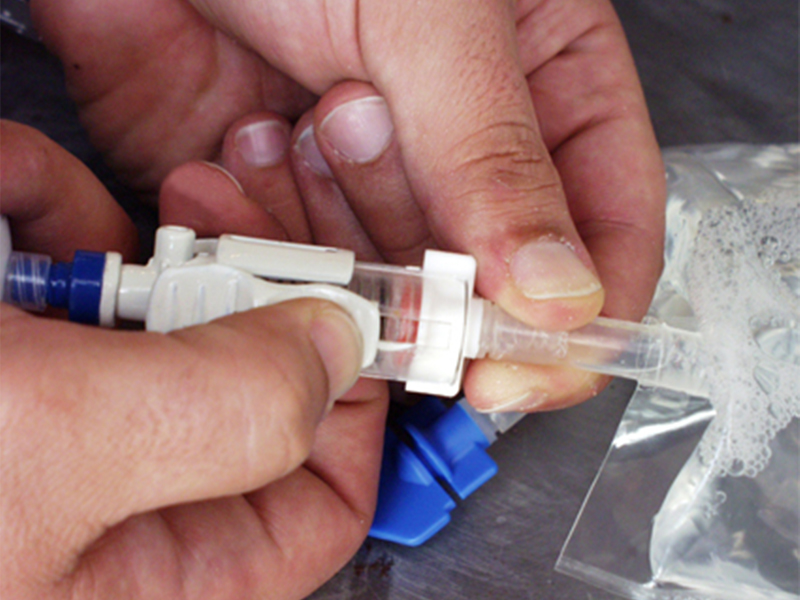
Fluid transfer between containers created several risks of drug exposure from needle-stick and splattering. Furthermore drips and splatters might result in the scrapping of other expensive drugs within the safety chamber.
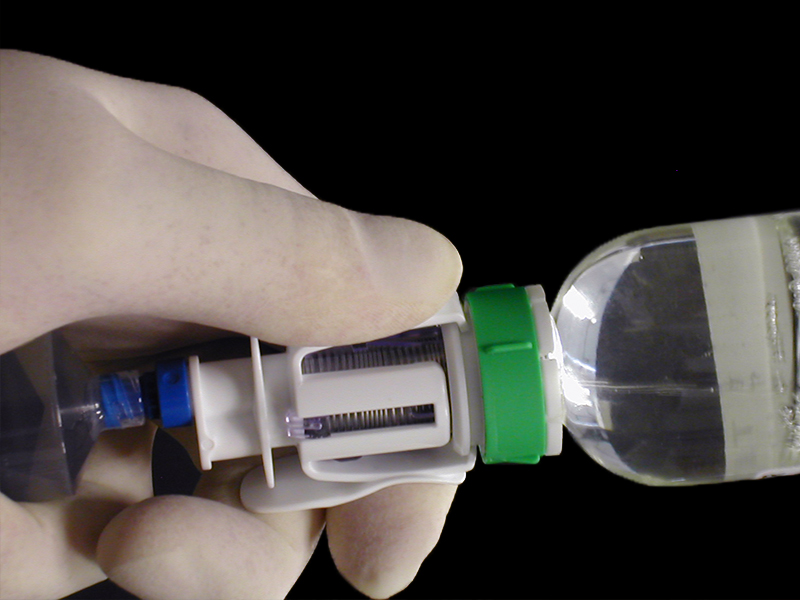
Guided by research insights, PDD designed a unique fluid transfer device that addresses major safety and practicality issues with the development of a telescopic needle mechanism with a self-sealing luer and a hydrophobic vent to prevent aerosolisation.
With this patented design, PDD helped Bristol Myers Squibb to expand stakeholder acceptance of Taxol® in oncology departments by making its journey from factory to patient easier and safer.