Innovation on a time limit
Responsiveness, along with the right capability and experience, is a key factor that medical device companies seek from their product development partners – no matter how lengthy are the regulatory routes.
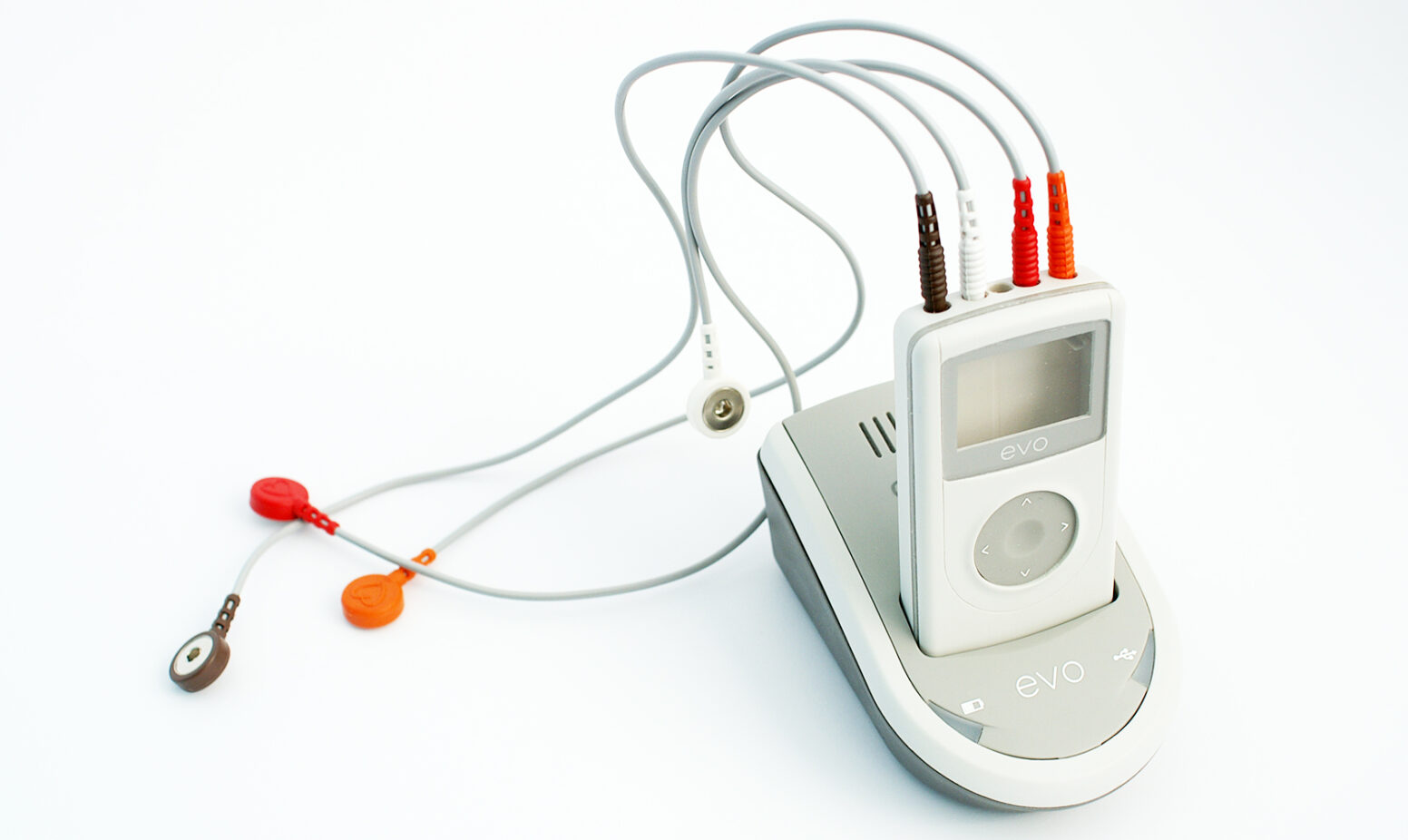
In the case of Spacelabs Healthcare, a pioneer in the cardiac diagnostics sector, responsiveness was especially needed. They set a goal to develop a state-of-the art, lightweight and easy-to-use ECG recorder within 6 months in order to launch at the American Heart Association exhibition.
PDD accepted the challenge to design the casing and user interface for the ambulatory recorder based on Spacelab’s core Holter technology. A docking station and holster were also commissioned to be developed within the timeframe. The ambulatory recorder needed to have a contemporary look and small enough to be discreet underneath clothing, which would improve compliance.


By working closely with our client and with constant communication between our engineers, designers, model-makers and external partners in Singapore, our concurrent development approach minimised risk whilst ensuring that solutions were commercially desirable for the end-user. Through rapid succession of concept & detailed engineering analysis, PDD produced and delivered prototypes for verification testing and usability validation while simultaneously starting tool design for injection mouldings.
PDD’s risk-managed, responsive and quality-focus approach enabled Spacelabs Healthcare to launch the evo ECG monitor successfully and quickly into the rapidly-developing telemedecine market.
PDD met the tight timescales imposed on this project and we have developed a good working relationship… We were impressed with the processes PDD implemented.